GENE-ENVIRONMENT INTERACTIONS
(A CHALLENGE FOR THE FUTURE)1
ROSIVAL, L., TRNOVEC, T.
Institute of Preventive and Clinical Medicine,
Bratislava, Slovak Republic
Corresponding author: Prof. L. Rosival, M. D., D. Sc.
Institute of Preventive and Clinical
Medicine
Limbová ul. 14, 83101 Bratislava,
Slovak Republic
Tel: 59369226
Fax: 54773906
CEJOEM 1999, Vol.5. No.1.:3-16
Abstract: Human health is determined by the interplay between
genetic factors and the environment. Scientists collaborate on studies
to understand the molecular and genetic problems of environmentally caused
diseases. The Environmental Genome project in the U.S.A. seeks to determine
genetic sequence diversity data for the U.S. population on more than 200
genes known to control susceptibility to environmentally linked diseases.
Studies at the molecular level in humans suggest that there is an individual
variability in genetic parameters, consistent with the differing susceptibility
to disease. More work is needed to assess genetic polymorphisms of key
enzymes, for the activation and detoxication of xenobiotics is a determining
factor for the susceptibility to environmental agents. Much can be learned
from the mechanisms, genetics, and epidemiology of environmental carcinogens.
The incorporation of biochemical, molecular, and cytogenetic probes (biologic
markers of exposure, effect and susceptibility) in epidemiologic studies
is of utmost importance. To assess the potential risk to individuals or
the population, more extensive knowledge is needed about the genetic background
and the interaction between genes and the environment. The complex interplay
between genes and the environment represents a great challenge to scientists,
and also an important opportunity to reduce the burden of disease and dysfunctions
to humans.
Key words: Genes, environment, genetic polymorphism, public health
INTRODUCTION
The changing world is experiencing changing
patterns of health. Influences include: rapid modernisation; an everyday
life dependent on technological progress; changing behaviour: sedentary
living, excessive or ill-balanced diets and smoking; and a deteriorating
environment: air pollution, exposure to chemicals and radiations, contamination
of soil and water, and hazards to food safety.
Human health is determined by the interplay
between genetic factors and the environment. Genes are only one part of
the disease picture. Many common diseases such cancer, asthma, osteoporosis
are known to have important environmental elements.
Many of the genes in the genome of humans
(human genome encodes for 50,000 to 100,000) influence the impact of environmental
agents on the organism. The exact number of genes involved in the organism’s
response to environmental factors is unknown but could be very large (Barrett
et al., 1997).
Genes control cellular differentiation,
division and death. When critical genetic material is altered, the functions
over which it exerts control can go away, leading to birth defects, cancer,
neurobehavioral abnormalities, and other diseases and dysfunctions. A better
understanding of these genes, the ability of environmental agents to interact
and damage them, the relationships between a xenobiotic’s chemical structure
and its binding affinity to critical cellular targets, and the consequences
of genetic malfunction (Olden, 1994) is needed.
Understanding the role of genes in human
disease will improve our understanding of genetic disease etiology as well
as our ability to predict. Insight into the genetic basis of chronic disease
etiology will have an immediate impact by suggesting novel therapeutic
approaches and aiding new drug discovery (Ellsworth et al., 1997).
The most important basis for these studies
is the Human Genom Project, which began in 1990 and is a co-operative multinational
initiative in the U.S.A. and has gained support with the biomedical science
community. The ultimate goal is
to determine the complete DNA sequence
of human genome as well as genomes of several model organisms: bacteria
(Escherichia coli), yeast (Saccharomyces cerevisiae), nematode (Caenorhabditis
elegans), fruit fly (Drosophila melanogaster), and mouse (Mus musculus).
Important similarities in chromosomal structure and gene function between
study organisms and humans will prove invaluable in the process of determining
gene functions and mechanisms of genetic disease etiology.
The contributions of genetic toxicology
to an integrated information system on genetics will facilitate the translation
of Human Genom Project into the practice of medicine and public health
in the 21st century (Khoury, 1997).
Progress on the Human Genome project has
led to an explosion of genetic information. Of the estimated 100,000 human
genes, more than 5,000 have been mapped to specific chromosomes (Khoury
and Dorman, 1998).
The keen interest in this project has several
reasons (Guengerich, 1998):
-
Identification of genes that show altered
expression levels in tumours.
-
There is a long history of interest in inherited
diseases.
-
If genetics contributes to rare diseases,
it should also contribute to more common diseases.
Studies on human drug metabolism over the
past 20 years have provided convincing evidence of the wide variability
in function of the enzymes of xenobiotic metabolism (e.g. cytochromes P450,
N-acetyltransferases, and glutathione S- transferases) and in vivo significance
of these variations in the disposition of these drugs in humans.
A large part of environmental health research
is focusing on identifying the environmental causes of disease (e.g. endocrine
disruptions and lead). Future research can help in understanding the molecular
and genetic basis of environmentally caused diseases. Polymorphisms in
Environmental Response Genes are depicted in Fig. 1.
(Albers, 1997).
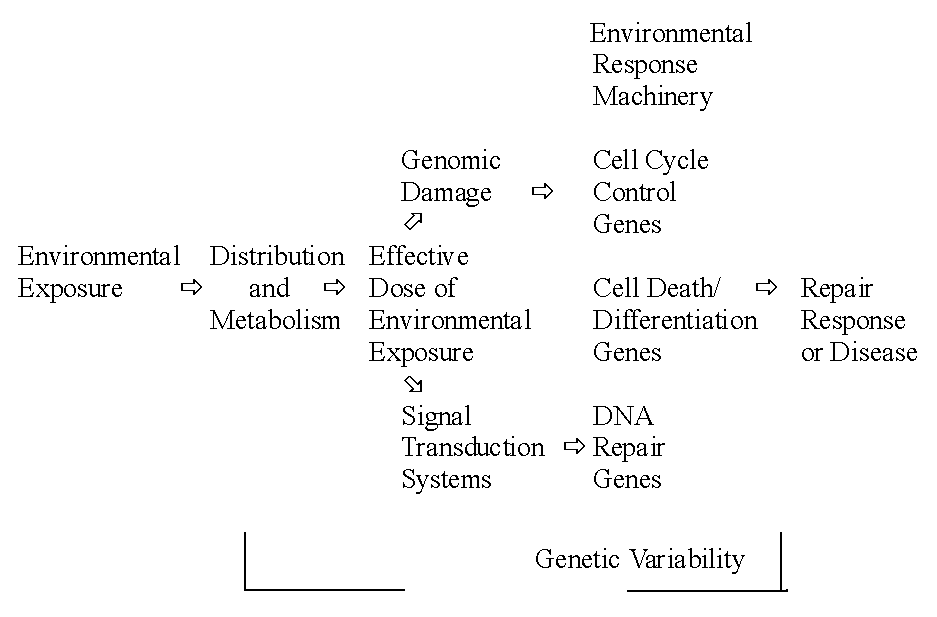
Fig.
1. Polymorphisms in environmental response genes can modify an individuals
risk for disease (Albers, 1997)
GENETIC POLYMORPHISM
Genetic polymorphism is a term used to describe
variants occurring at an incidence of >
1%. Polymorphisms are common among humans. Some have no functional significance,
and the identification of which do and which do not have any functional
significance will be necessary in order to be able to interpret the information
obtained in the Environment Genome Project (Guengerich, 1998).
Polymorphisms often affect the functions
of genes but some may change the level of expression of a gene or change
the activity of a gene product, for example, an enzyme (Barrett et al.,
1997).
Most of the approximately 50 known inherited
traits that could potentially enhance an individual’s susceptibility are
often difficult to detect. Many genetic conditions associated with enhanced
susceptibility to environmental chemicals remain to be discovered. As human
population is biologically diverse and genetically heterogeneous, it is
not surprising that differences in susceptibility to disease among individuals
with or without exposure to environmental chemicals exist.
Studies at the molecular level in humans
suggest that there is a wide interindividual variability in genetic parameters,
consistent with this differing susceptibility to disease.
Genetic variations among individuals represent
a basic attribute of living matter (e.g. blood groups, enzyme variants,
protein variants, chromosomal variants). It appears that the genetic diversity
of the three major human races (Caucasian, Mongoloid and Negroid) is much
smaller than the average heterozygosity (Goedde, 1986) and ethnic differences
in reactions to drugs and xenobiotics have also been reviewed.
Effects of genetic variation on susceptibility
to occupational and environmental chemicals is drawn primarily from the
differences observed in the response to drugs (Vesell, 1987).
It has long been suspected that genetic
factors affect susceptibility to occupational diseases. It must be appreciated
that this response is modified by many other host (e.g. nutrition, health
status, gender, life-styles) and environmental factors (e.g. coexisting
exposures) (Table 1).
Enhanced susceptibility to occupational
and environmental chemicals has clinical implications (observation of the
susceptibility of workers and the vulnerability of the foetus, the neonate,
the elderly, and those who are ill) implications related to administered
drugs and regulatory implications (the issue of special susceptibility
in the standard-setting process for air and water pollutants). A more specific
and consistent approach is needed in the formulation of environmental policy
and the setting of standards for pollution control in children (especially
air and water pollutants and pesticide residues in food).
The fields of human genetics and epidemiology
used to be independent disciplines with very little interaction between
them (Ellsworth et al., 1997). Genetic concepts were integrated with epidemiologic
methods to capitalise on the advantages of their perspectives. The greatest
benefit to public health from genetic epidemiology research will come from
the better understanding of the genetic etiology of the common chronic
diseases (e.g. coronary artery disease and diabetes) and the common forms
of cancer (such as breast and colon cancer) and from gene-environment interactions.
It can be seen that epidemiology has moved
away from a search for explanation of diseases at the population level
to search at the individual level. This means emphasis on the inherited
factors in common diseases. Placing undue emphasis on them does not comport
with scientific realities (e.g. no more than 5 percent of all cancer incidences
can be attributed to single inherited factors – Trichopoulos et al., 1996).
This may also impede efforts to understand
the complex causes of the disease. There is a pressing need for research
on the sensitivity and predictive value of genetic tests, the efficacy
of efforts to prevent the occurrence of or treat the resulting disorders
and the development of adequate informed consent procedures (Holtzman and
Andrews, 1997).
Different visions of the future of public
health are influenced by a combination of medical, technological, social-economic
and political factors. New gene discoveries require that the public health
community should take a leading role in translating the results of these
discoveries into effective strategies to prevent epidemies and disability
in the population by targeting environmental, behavioural and medical interventions
to each person’s genetic susceptibility. (Khoury, 1997). Table
2 shows that genetic epidemiology will play a central role in providing
data that will be useful for evaluating disease risks, developing sound
health policies and providing valuable information both for professionals
and the public.
TABLE 1. Genetic Factors and Susceptibility
to Occupational and Environmental Chemicals (Tarcher and Calabrese, 1992)
|
Predisposing factor
|
Incidence
|
Chemical(s)
|
Status of genetic-
environmental
interaction
|
| Glucose-6-phosphate
dehydrogenase deficiency |
About 12%
among African-American males; very high in tropical and subtropical countries |
Oxidizing
chemicals |
Likely |
| Sickle-cell
trait |
7–13% among
African–Americans; 30% of population in parts of Africa |
CO, aromatic
amino compounds |
No clear
evidence |
| Methaemoglobin
reductase deficiency |
About 1%
of population are heterozygotes |
Nitrites,
aniline |
Definite |
| Aryl hydrocarbon
hydroxylase induction |
High-induction-type
Caucasians about 30% |
Polycyclic
aromatic hydrocarbons |
Possible |
| Slow acetylator
phenotype |
Caucasians
and Negroes about 60%; Orientals about 10–20% |
Aromatic
amine-induced cancer |
Possible |
| Paraoxonase
variant |
Caucasians
about 50%, Orientals about 30%, Negroes about 10% |
Parathion |
Possible |
| Acatalasia |
Mainly Japan
and Switzerland, reaching 1% in some areas of Japan |
Hydrogen
peroxide |
Definite |
| Nontaster
status |
30% Caucasians,
10% Chinese, 3% Negroes |
Goitrogens
(thiourea, etc.) |
Definite |
| a1-Antitrypsin
deficiency |
Homozygotes
about one in 6700 North American Caucasians |
Respiratory
irritants Smoking |
Most likely
definite |
| Immotile
cilia syndrome |
About 1:40,000
in all major races |
Respiratory
irritants, smoking |
Most likely |
| Immunologic
hypersensitivity |
Unknown,
2% in some occupational populations |
Isocyanate |
Definite |
The incorporation of biochemical, molecular, and cytogenic probes (termed
biomarkers) into epidemiology overcomes the limitations of classical epidemiology
(the frequent lack of exposure assessment data and the long delay between
toxic exposure and the appearance of symptoms).
TABLE
2. The impact of genetic epidemiology on the future of public health
(Khoury, 1997)
-
Will provide data on the public health impact of human genes and their
interaction with preventable risk factors on disease morbidity, mortality
and disability in various populations.
-
Will provide data to health policy guidelines
on the appropriate use of genetic testing in disease prevention and public
health programmes.
-
Will provide data to evaluate the impact of
population based prevention programmes that reduce morbidity and disability
associated with disease genes.
-
Will provide data on the laboratory quality
of genetic testing.
-
Will become increasingly needed in core training
programmes in epidemiology and public health.
-
Will provide more qualitative disease genetic
risk information in integrated and online genetic information systems used
by medical and public health professionals and the public.
|
BIOMARKERS
Biomarkers in the context of environmental health are indicators of events
in biological systems and samples. Fig. 2 shows the progression
from exposure to clinical disease (Jendrychowski and Goldsmith, 1992)
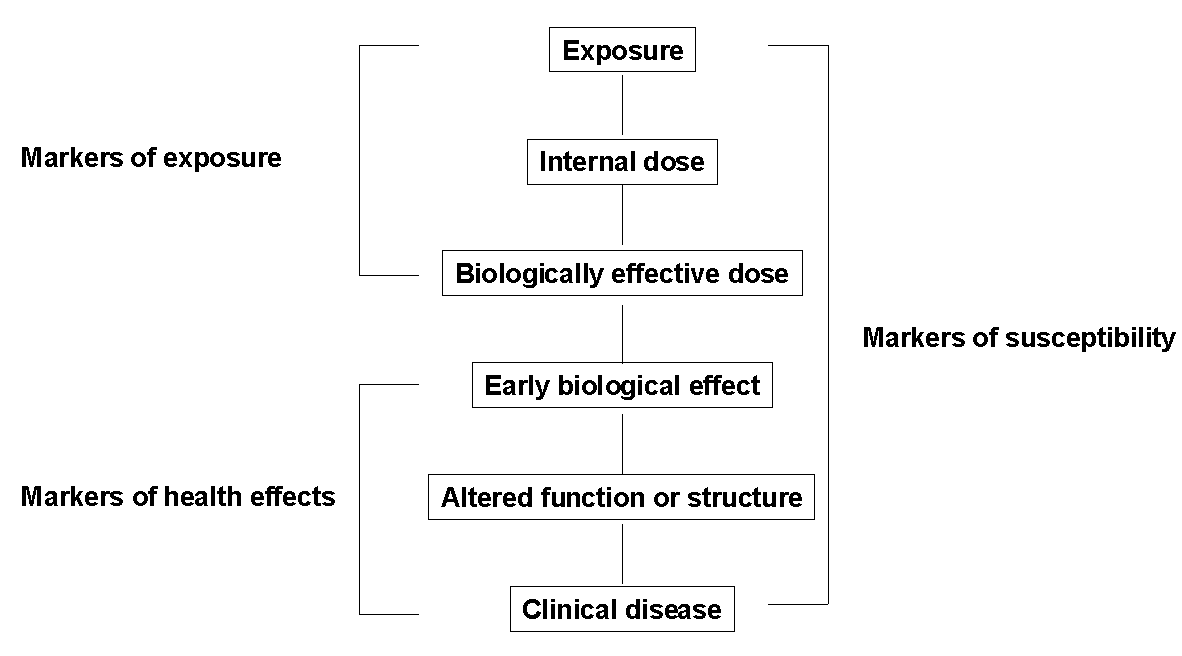
Fig. 2. Kinds of Biological Markers (Jendrychovski
and Goldsmith, 1992)
These biomarkers can be divided into markers of exposure, markers of
effect, and markers of susceptibility.
Biomarkers of Exposure
These biomarkers can be divided into internal dosimeters and biologically
effective dose. They are connected with several different types of exposure.
In our studies foetal exposure to chlorinated pesticides has been shown
to correlate with embryonal measurements of chlorinated pesticides (Rosival
et al., 1983). A higher level of placental contamination by organochlorine
compounds was observed in an industrial locality as compared to a similar
or even higher placental contamination by heavy metals in a rural area.
Histochemically, the presence of heavy metals in
the placenta could be detected, in particular in the syncytiotrophoblast
(Reichrtová, 1995). This refers also to other chlorinated substances
(e.g. PCBs).
Measurement of bone lead in epidemiologic studies has proved to be
useful in exposure assessment studies, i.e., in spotting factors that contribute
most to retained body lead burden, and in investigating cumulative lead
exposure as a risk factor for poor health outcomes such as hypertension,
kidney impairment, cognitive impairment, behavioural disturbances, and
adverse reproductive outcomes (Hu, 1998).
Biological Tolerance Values (BAT) are defined as
maximum permissible quantity of a chemical compound, its metabolites, or
any deviation from the norm of biological parameters effected by these
substances in occupationally exposed persons. They are established, as
a rule for blood and urine, the knowledge of action mechanisms is needed.
Examples of biological monitoring for selected agents, e.g. lead (lead
in blood and urine ZPP, zinc protoporphyrin), cadmium (cadmium in urine
or blood), mercury (mercury in urine), organophosphates (red cell or serum
cholinesterase activity), benzene (total phenol or muconic acid in urine,
benzene in expired air), trichlorethylene (TCE), trichloracetic acid in
urine (TCA) (trichloroacid in urine, TCE in end-expired air). Biological
monitoring follows several goals: the detection of exposed workers at an
early stage before significant adverse effects have occurred; quantifying
or classifying exposure for epidemiologic studies and identifying variations
in susceptibility with workers and population.
Biomarkers of Effect
These biomarkers can be categorised based on their relationship to health
status – from normal health, to health impairment, or to overt disease
(Bearer, 1998).
There are many biomarkes of effect, e.g. hematological
toxicity, immunotoxicity, reproductive toxicity (International Programme
on Chemical Safety, 1993). Regarding other biomarkers of effect entails
this group also biomarkers at the genomic level, e.g. cytogenetic markers
(Evans, 1984), chromosome aberrations (Evans, 1984), sister chromatid exchange
(Tucker et al., 1993), micronuclei (Heddle et al., 1991). Markers of gene
expression include assays for detection of mRNA or for detection of proteins
(Barrett et al., 1997). Methodologies for examining gene expression by
the detection of the encoded proteins all depend on the development of
antibodies that are theoretically specific for the identification of the
protein of interest (Barret et al., 1997).
In a study directed towards the documenting of the
possibilities offered by the detection of genetic changes in human populations,
the Comet Assay technique was used. Workers exposed to polycyclic aromatic
hydrocarbons at a chemical plant showed significantly increased numbers
of DNA breaks as compared to two control groups. The results have confirmed
that this method is suitable for the monitoring of biological responses
to occupational exposure (Šrám, 1998).
The Comet Assay has been modified by incubating
DNA of individual cells with specific repair enzymes which detect certain
types of DNA lesions (Collins et al., 1995). This way, the method is not
only suitable for the detection of direct breaks but also of other types
of DNA lesions, in particular oxidative DNA lesions and large DNA adducts.
This significantly extends the number of applications of the method in
research, in particular concerning genotoxicology, molecular epidemiology,
diagnosis and therapy as in studying basic processes in the cell.
Potential uses of biomarkers of effect are of disease
prognosis, and as adjuncts to other biomarkers in providing refinements
of epidemiology and risk assessment.
Most of the biological biomarkers of effect especially
at the genomic level are still experimental tools whose utility remains
to be established.
Biomarkers of susceptibility
A marker of susceptibility is an indicator of an inherent or acquired limitation
of an organism’s ability to respond to the challenge of exposure to a specific
xenobiotic substance. Some people are susceptible because of inborn differences
of metabolism, physiological characteristics, their nutritional status,
or absorption characteristics. For example, the measurement of airway reactivity
to inhaled bronchoconstrictors can be used as a biomarker of susceptibility.
Increased non-specific airway reactivity is a characteristic of most asthmatics
and can play a role in disease activity. This marker can also relate to
induced variations in absorption, metabolism, and response to environmental
agents.
The concept of biomarkers of susceptibility encompasses enzymes of
activation and detoxification, repair enzymes, and changes in target molecules
for toxic chemicals. Objectives of use are as follows: evaluation of interindividual
variation, repair enzymes, and changes in target variation of risk, determination
of the role of genetic variations and improvement of the detection of environmental
hazards.
Methods for studying phenotyping expression concern
the use of probe drugs in vivo (metabolic polymorphisms can be identified
by determining the metabolic ratio) and in vitro. There are also other
methods (polymorphisms of xenobiotic-metabolizing enzymes can be detected
at other phenotypic levels). Variations in the activities of the xenobiotic
metabolising enzymes by polymorphic changes can influence the genotoxic
effects.
According to Barrett et al. (1997) the following
recommendations are made: phenotyping should be continued along with genotyping,
the combined impact of all relevant genes for a given exposure needs to
be assessed, better kinetic characterisation of enzyme substrate and inhibitor
specificity must be determined, a better understanding of the regulation
of enzyme expression by environmental agents is needed, the value of intermediate
markers of exposure such as DNA adducts should be studied. A future approach
will be to use information obtained from analysis populations of interethnic
groups.
The identification of valid biomarkers that indicate
exposure, effect, or susceptibility is a complicated process involving
studies in animals, refinements in laboratory assays, and studies in special
human populations e.g. workers and children.
Validation and use of biomarkers in such areas as
asthma, respiratory disease, cancer, and neurodevelopmental effects would
hasten progress in understanding modes of exposure and risk assessment
for children (Bearer, 1998). Other examples are biomarkers of leukaemia
risk: benzene as a model (Smith and Zhang, 1998), biomarkers for assessing
human female reproductive health (Lasley, and Overstreet, 1998), and bone
lead as a new biomarker of lead dose (Hu, 1998).
An integral part of the use of biomarkers of susceptibility
are ethical, social and legal issues surrounding studies of susceptibility
(e.g. genetic screening of workers, the right of the subjects to be informed,
confidentiality, duties of the research, etc.). This problem raises further
questions as follows (Loffredo et al., 1998). Should employers be able
to transfer chemically sensitive workers to jobs with lower exposure levels
rather than reducing exposure levels to a safe level for all? Could employers
expose more resistant workers to higher exposure level? In cases of alleged
chemical injury, would lawyers misuse knowledge of genetic susceptibilities?
It is a comprehensive and extremely sensitive issue and technological progress
continues to challenge our sense of what may be deemed “wrong” or “morally
appropriate”, respectively.
In order to translate the results of the genetic
research into opportunities for treating and preventing disease and promoting
health, population-based epidemiologic studies are increasingly needed.
Human genome epidemiology is an intersection between molecular epidemiology
and genetic epidemiology. The topics addressed range from population-based
epidemiologic research on gene variants to the evaluation of genetic test
services (Khoury and Dorman, 1998).
One of the important goals of human genome epidemiology
is to assess the magnitude of disease risk associated with gene-gene and
gene-environment interactions in different populations. The consequence
of this approach is the Environmental Genome Project (EGP). It is an activity
of the National Institute of Environmental Health Sciences (NIEHS) in the
U.S.A. to characterise the variations in important human genes and to relate
these differences to the susceptibility of humans to chemical and physical
agents (e.g. ionising radiation) in the environment.
The main goal of EGP is to enhance population-based
research toward identifying environmental exposure-disease relationships.
This will lead also to prevention and intervention strategies in combination
with primary prevention measures (reducing or eliminating of environmental
exposures). In connection with this further identification of the non genetic
factors — occupational, environmental and food exposure, life styles and
infectious diseases that influence the hypothesis whether the altered susceptibility
genes lead to disease — is a great potential to prevention.
The Environmental Genome Project, which is to complement
the Human Genome Project seeks to determine genetic sequence diversity
data for the U. S. population on more than 200 genes known to control susceptibility
to environmentally linked diseases, and to develop a central database of
polymorphisms of these genes (Albers, 1997). On the basis of epidemiological
studies, it will be determined how polymorphisms account for the increased
risk of or resistance to disease upon exposure to specific chemicals. The
project will also look at environmental factors ranging from diets to effects
of chemical mixtures.
According to Olden´s estimation (Albers, 1997)
more than 200 candidate genes are under consideration for the study from
several broad classes.
It concerns genes controlling the distribution and
metabolism of chemicals, genes for the DNA repair pathways, genes for the
cell cycle/cell death control system including apoptosis, genes for metabolism
of nucleic acid precursors, and genes for signal transduction systems controlling
the expression of genes in other classes.
About 100 candidate genes (metabolism and detoxification) regulate
cytochrome P450s, N-acetyl transferase, glutathione S-transferases, glucuronyl
tranferases, sulphotransferases, metallothioneins, and variants of the
enzyme paraoxonases (exposure to organophosphates) with difficulty in breaking
down nerve gas sarin and related organophosphates. Variations in alcohol
dehydrogenase genes have been linked to alcohol-related diseases and cancer.
There are many more genes that can be tested but there are great economic
constraints (e.g. the cost to sequence the “environmental” genes in 1,000
people will be about $ 60 million). Perhaps the greatest advances have
developed from the associations between exposure, polymorphisms in carcinogen-metabolising
genes (Suk and Collman, 1998). The important enzymatic pathways involved
in carcinogen metabolism concern two categories: oxidation, reduction,
and hydrolysis are termed phase I reactions, whereas conjugation and synthesis
are phase II reactions.
Phase I enzymes mainly involve the cytochrome P450 group, but also
include N-acetyltransferase, important in metabolic activation. Phase II
enzymes (e.g. glutathione S-transferases) are involved in detoxification
reactions and help the body dispose of carcinogens in lung, brain, and
bladder cancers, and other diseases. Another very common phase II reaction
concerns sulfotransferases to form another common phase II reaction for
phenols is the conjugation with sulfate to form sulfate monoesters. Methylation
is generally not a quantitatively important metabolic pathway for xenobiotics,
but is an important pathway in the intermediary metabolism of both N- and
O- containing catechols and amines (Borchardt,1980).
Among the most studied genotypes are human glutathione
transferases especially in relation to the genetic polymorphism of glutathione
5-transferase M 1 (GSTM 1).
The effect of GSTM 1 and N-acetyl transferase 2
was seen in coke workers on mutagenicity of urine and of glutathione S-transferase
T 1 on the chromosomal aberrations in subjects from the 1.3-butadiene monomer
production unit. Effects of genotypes on DNA adducts were found from lung
tissue of autopsy donors and from placentas of mothers living in an air-polluted
regions, protein adducts in smokers, SCE in smokers and non smokers and
Comet Assay parameters in postal workers (Šrám, 1998).
If the genetic polymorphism responsible for detoxification
pathways is known it is reasonable to suggest that subjects lacking these
genes should not be employed in occupations in which certain types of exposures
are likely to occur. Ethical questions must be addressed if knowledge about
individual genotypes is to be used to prescribe preventive measures among
specific groups.
Regarding methods for studying genetic polymorphisms,
identification of novel genetic polymorphisms and genotyping of populations
is of great importance.
Advances in the areas of molecular genetics and
molecular epidemiology now make it possible to study genetic polymorphisms
of the enzymes used to metabolise xenobiotic compounds, presymptomatic
genetic changes (e.g. somatic mutations) associated with increased risk
disease, and other genetic markers of susceptibility. The capability studying
the RNA species present in the tissue samples permits to study tissue specific
gene expression. The advent of new technologies, polymerase chain reaction
(PCR) now makes it possible to detect specific genotypes and specific RNA
species in extremely low concentrations. PCR technology alone greatly increases
the potential of obtainable data from very small amounts of banked tissue
of any type as long as its preparation and preservation conditions are
compatible with PCR analysis (Lee et al., 1995).
An Environmental and Biological Specimen Bank was
established in 1998 in the premises of the Institute of Preventive and
Clinical Medicine in Bratislava (Slovak Republic). This is the first Specimen
Bank in Central and Eastern European Countries. Its aim is to provide a
broadly acknowledged basis for environmental and biological monitoring,
identification of trends in environmental pollution, as well as to identify
new environmental chemicals. The development of sample banks is also important
to speed the identification and validation of markers.
As for ambient air pollution Whyatt (1998) measured
the amount of PAHs bound to DNA (PAH-DNA adducts) in maternal and umbilical
blood cells. Results indicate, that PAH-induced DNA damage in mothers and
newborns is increased by ambient air pollution. In the fetus, this damage
appears to be enhanced by the P450A1 (CYP1A1) Mspl polymorphism. Genetic
damage in newborns associated with environmental PAHs raises concern about
carcinogenic risks from in utero exposure to this widespread contaminant.
With regard to other candidate genes there are about
50 DNA repair genes. The inability to repair such mistakes can cause skin
and other cancers.
Chemical receptor genes include ones that modify
pathways related to toxic response, e.g. Ah receptors, estrogen receptors,
progesterone receptors, and endocrine disrupter pathways.
Variations in genes in neurotoxicology, reproductive, and developmental
toxicology are also strong candidates.
Genes involved in nutrition pathways or the metabolism of nutrients
(e.g. vitamins and minerals) account for another 25 candidates. Certain
polymorphisms in nutrient pathways contribute to diseases such as cirrhosis
and liver cancer. Steroid metabolism genes involved in the synthesis of
estrogen, progesteron, and testosterone account for some 25 candidates
(Albers, 1997).
Characterisation of the variability in response
to a contaminant requires the analysis of several susceptibility factors.
Recent results in determining genetic susceptibility are discussed by Suk
and Collman (1998) and Whyatt at al., (1998). Lead constitutes a rare example
where the dose-response relationship has been thoroughly studied epidemiologically.
In children lead exposure is manifested in impaired behavioural, cognitive,
and motor functions (Grassman, 1996). Currently, deficits are thought to
occur with blood lead levels (BLLs) below 10 µg/dl (Šov?ikova et
al., 1997), and the calculated threshold of less than 1 µg/dl (Schwartz,
1994).
A specific example of research in the characterization
of a genetic polymorphism of a commonly occurring gene now leads us to
begin to understand the relationship between lead exposure levels and cognitive
impairment in susceptible subpopulations in children. An enzyme of the
heme biosynthesis pathway, delta-aminolevulinate dehydratase (ALAD) is
a protein that is encoded by a gene in the 9 q 34 chromosome locus. It
is polymorphic in the population, with two common alleles, ALAD-1 a ALAD-2.
This structure results in three distinct genotypes, ALAD 1-1 1-2, and 2-2,
which are distributed in the population (Suk and Collman, 1998). It is
hypothesised that individuals with the ALAD-2 allele could be more susceptible
to lead exposure if the ALAD subunit binds lead more tightly ALAD-1 subunit
(Astrin et al., 1987). Individuals with the ALAD 1-2 and 2-2 allele might
have higher blood lead concentrations as well as higher total body burden,
making them more likely to show clinical and subclinical manifestations
of low-level exposure (Suk and Collman, 1998).
Interactions between the ALAD polymorphism, developmental
stage, dietary deficiencies, and gender are possible. As a result, the
most susceptible population cannot be identified.
Genetic polymorphisms in metabolic enzyme activity
add an important dimension of variability. Depending on the particular
chemical, this may serve as a protective factor of increased susceptibility
to toxic effects (e.g. epoxide hydrolyse and fetal hydantoin syndrome —
Graeter and Mortensen, 1996).
Response variability can be analysed in large cohorts
with uniform exposure. Future approaches to risk assessment may employ
mechanistic models where the variability in the intermediate steps between
exposure and health outcome are modelled using a combination of animal
and human in vivo and in vitro endpoints.
CONCLUSIONS
Despite recent extraordinary advance in cellular and molecular biology
that has enhanced our understanding of the basis for human disease, the
approaches for incorporating this information into risk assessment and
risk management activities are still in their infancy (Preston, 1996).
Determining how to incorporate the influence of genetic susceptibility
and sensitivity into the process is particularly difficult. Therefore the
projects including gene-environment interactions have great public health
implications. It is the basis for understanding how chemical agents interact
and providing data to prevent and not just treat final-stage disease.
A great challenge not only to EGP, but also to other
activities regarding genetic problems and human health is in the area of
their ethical, legal, and social implications. These issues are complex
and many-layered and we should foster an open dialogue on these implications
with both scientific and non-scientific communities.
The complex interplay between genes and environment
represents also a great challenge to scientists, and it is also an important
opportunity to reduce the burden of disease and disfunctions on humans.
REFERENCES
ALBERS, J. W. (1997). “Understanding Gene – Environment.” Environ.
Health Perspect. 105: 578–580.
ASTRIN, K. H. et al. (1987). “Delta-aminolevulinic acid dehydratase
isozymes and lead toxicity.” Ann. N. Y. Acad. Sci. 514: 23–29.
BARRETT, J. C. et al. (1997). 12th Meeting of the Scientific Group on
Methodologies for the Safety Evaluation of Chemicals: Susceptibility to
Environmental Hazards. Environ. Health Perspect. 105: Suppl. 4, 694–737.
BEARER, C. F. (1998). “Biomarkers in pediatric environmental health:
A cross-cutting issue.” Environ. Health Perspect. 106: Suppl. 3, 813–816.
BORCHARDT, R. T. (1980). “N- and O-methylation.” In: Enzymatic Basis
of Detoxication. (J. B. Jacoby, ed.) Academic Press, New York, pp. 43–62.
COLLINS et al. (1995). “The comet assay modified to detect DNA base
oxidation, and repair of DNA damage in cellular and subcellular systems.”
J. Cell. Biochem. (Suppl), 347.
ELLSWORTH, D. L. et al. (1997). “Impact of Human Genome Project on epidemiological
research.” Epidemiol. Rev. 19: 3–13.
EVANS, H. J. (1984). “Human peripheral blood lymphocytes for the analysis
of chromosome abberations in mutagen tests”. In: Handbook of Mutagenicity
Test Procedures, 2nd ed. (B. J. Kilbey, M. Legator, W. Nichols, C. Ramel,
eds.) Elsevier Amsterdam, pp. 405–427.
GOEDDE, H. W. (1986). “Ethnic differences in reactions to drugs and
other xenobiotics: Outlook of a geneticist”. In: Ethnic Differences in
Reactions to Drugs and Xenobiotics (W. Kalow, H. W. Goedde, D. P. Agarwal,
eds). Alan R. Liss, New York, pp. 9–14.
GRAETER, L. J., and MORTENSEN, M. E. (1996). “Kids are different: developmental
variability in toxicology.” Toxicology. 111: 15–20.
GUENGERICH, F. P. (1998). “The Environmental Genome Project: Functional
analysis of polymorphisms.” Environ. Health Perspect. 106: 365–368.
HEDDLE, J. A. et al. (1991). “Micronuclei as an index of cytogenetic
damage: past, present, and future.” Environ. Mol. Mutagen. 18: 277–291.
HOLTZMAN, N. A., and ANDEREWS, L. B. (1997). “Ethical and legal issues
in genetic epidemiology.” Epidemiol. Rev. 18 : 163–174.
HU, H. (1998). “Bone lead as a new biologic marker of lead dose: Recent
findings and implications for public health.” Integrated Approaches for
Studying Hazardous Substances. 106: Suppl. 4, 961–967.
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY (1993). “Biomarkers and risk
assessment concepts and principles”. In: Environmental Health Criteria
155. World Health Organization, Geneva.
JENDRYCHOWSKI, W., and GOLDSMITH, J. (1992). “Health effects of environmental
contaminants.” In: Kryzanowski, J. K. (ed.) Seminars of Environmental Epidemiology.
WHO. Bilthoven. pp. 7–33.
KHOURY, M. J. (1997). “Genetic epidemiology and the future of disease
prevention and public.” Epidemiol. Rev. 19: 175–180.
KHOURY, M. J., and DORMAN, J. S. (1998). “The Human Genome Epidemiology
Network.” Am. J. Epidemiol. 148: 1–3.
KÖTELES, G. J. (1998). “The human lymphocyte micronucleus assay.”
Centr.
Eur. J. Occup. Environ. Med. 2: 12–30.
LASLEY, B. L., and OVERSTREET, J. W. (1998). “Biomarkers for assessing
human female reproductive health.” Integrated Approaches for Studying Hazardous
Substances. 6. Suppl 4, 955–960.
LEE, L. W., GRIFFITH, J., ZENICK, H., and HULKA, B. S. (1995). “Human
tissue monitoring and specimen banking: Opportunities for exposure assessment,
risk assessment, and epidemiologic research.” Environ. Health Perspect.
103: Suppl. 3, 3–8.
LOFFREDO, CH. A. (1998). “The Environmental Genome Project: Suggestions
and concerns.” Environ. Health Perspect. 106:A 368.
OLDEN, K. O. (1994). Vision for the Future. National Institute of Environmental
Health Sciences. USA.
REICHRTOVÁ, E. (1995). “Human placenta quality in ecologically
different regions. Part 2-P Placental structural changes and cord blood
IgE level.” Toxicol. Letters 78 (Suppl.):69–70.
ROSIVAL, L. et al. (1983). “Transplacental passage of pesticides into
human embryo.” Czechoslovak Medicine 5: 1–7.
SCHWARTZ, J. (1994). “Low-level lead exposure and children’s IQ: a meta-analysis
and search for a threshold.” Environ. Res. 65:42–55.
SMITH, M. T., and ZHANG, L. (1998). “Biomarkers of leukemia risk: Benzene
as a model.” Integrated Approaches for Studying Hazardous Substances 106:
Suppl. 4, 937–946.
SOVCIKOVA, E. et al. (1997). “Effects on the mental and motor abilities
of children exposed to low levels of lead in Bratislava.” Toxic Subst.
Mech. 16:221–236.
ŠRÁM, R. J. (1998). “Effect of glutathione S-transferase M1 polymorphisms
on biomarkers of exposure and effects.” Environ. Health Perspect. 106:
Suppl. 1, 231–239.
SUK, A., and COLLMAN, G. W. (1998). “Genes and the environment: Their
impact on children’s health.” Environ. Health Perspect. 106: Suppl. 3,
817–820.
TARCHER, A. B., and CALABRESE, E. J. (1992). “Enhanced susceptibility
to environmental chemicals.” In: Principles and Practices of Environmental
Medicine. (Tarcher, A. B., ed.) Plenum Medical Book Co. New York, London,
189–213.
TRICHOPOULOS, D. et al. (1996). “What causes cancer?” Sci. Am. 88:496–509.
TUCKER, J. D. et al. (1993). “Sister-chromatid exchange: Second report
of the Gene-Tox program.” Mutat. Res. 297:101–180.
VESELL, E. S. (1987). “Pharmacogenetic perspectives on susceptibility
to toxic industrial chemicals.” Br. J. Ind. Med. 44:505.
WHYATT, R. et al. (1998). “Relationship between ambient air pollution
and DNA damage in polish mothers and newborns.” Environ. Health Perspect.
106: Suppl. 3, 821–826.
Received: 30 December 1998
Accepted: 26 February 1999
Posted: December 1999 |
| Back |
|
|


