Editorial Review
The Low Dose Dilemma
G. J. Köteles
“Fodor József” National Center for Public Health, “Frederic Joliot-Curie”
National Research Institute for Radiobiology and Radiohygiene, Budapest,
Hungary
Corresponding author: Professor G. J. Köteles, MD, PhD, DSc, Director
“Frederic Joliot-Curie” National Research Institute for Rabiobiology
and Radiohygiene
H-1775 Budapest, POB. 101. Hungary
Phone/Fax: 36-1-226-0026
CEJOEM 1998, Vol.4. No.2.:103-113
Key words: Ionizing radiation, low dose, biological effects,
cellular effects, epidemiological effects
Introduction
The increasing use of ionizing radiations and nuclear energy all over the
world induces an ever-increasing interest of the professionals as well
as of the whole society in health protection and the risk due to these
practices. Many international and national bodies and organizations are
involved in the development of radiation protection philosophy, regulations
for the safe use of radiations and the practical implementations of rules.
One of the main forums devoted to the radiation protection is the International
Commission on Radiological Protection (ICRP) founded in 1928. The Commission
and its Committees have a leading role in elaborating the relevant recommendations.
The latter are usually considered by other international, including intergovernmental
organizations, scientific societies and last but not least by national
authorities responsible for legislations. In the exponentially growing
professional literature plenty of factual information, models and ideas
have been accumulated in recent years concerning the biological effects
of ionizing radiation including possible detrimental effects on human health.
For the sake of safety the international radiological protection and dose
limitation systems recommend their standards based on biological observations,
experimental and epidemiological data (ICRP 1991; IAEA-IBSS 1996). In the
trend of lowering the dose limits, however, the attributable risk to health
might even get lower than in several other industrial or other activities
of a civilized society. It also has to be noted that the implementation
of increasing safety results in considerable financial burden to the national
economies. Therefore, a rather wide discussion developed on the crucial
points of radiation biology and radiation protection whether it is justified
to assess the health risks by linear extrapolation of effects from large
doses to low doses (Tubiana 1991, Gonzalez 1994, Streffer and Tanooka 1996;
Duport 1996; Mossman et. al. 1996; IAEA 1997). Beside the scientific discussions
it has also to be considered that there is a deepening gap between the
risk assessment of the professionals and the risk perception of various
groups of the society (UNEP 1985; Faragó and Engländer 1987;
Köteles 1996).
The intention of the present review is to assist
those who are interested but could not follow these emerging ideas. Accordingly,
the review outlines the so-called dose-response models, the “low dose”
levels, lists the main pro and contra arguments concerning the validity
of linear-non threshold (L-NT) model of stochastic biological effects,
and points out a few examples on the cellular reactivities at low doses.
The advantages of maintaining the present view on the L-NT model as recommended
by the ICRP and accepted widely are also raised.
The features of the main dose-response relationships
The biological effects of ionizing radiation for radiation protection considerations
are grouped into two categories: the deterministic and the stochastic ones
(Fig. 1).
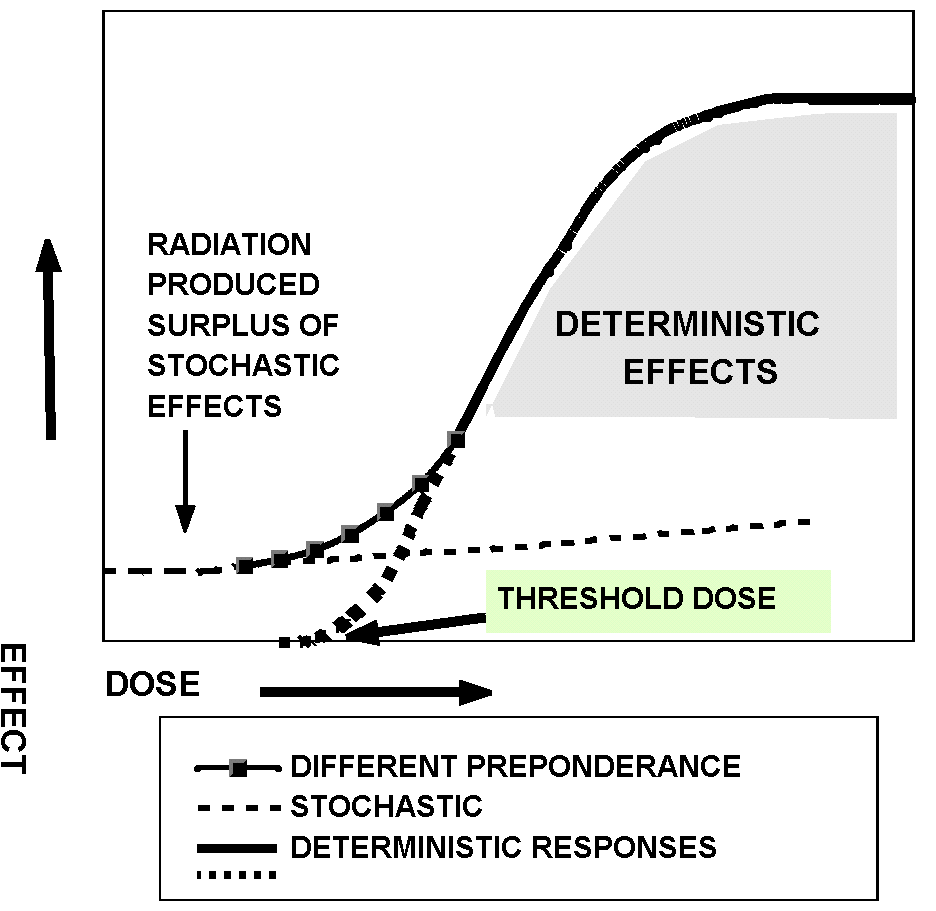 Fig. 1. Schematic dose-response curves for the stochastic and
deterministic effects of ionizing radiation
Fig. 1. Schematic dose-response curves for the stochastic and
deterministic effects of ionizing radiation
The deterministic effects occur when above a certain “threshold”
an appropriately high dose (above 500–1000 mSv) is absorbed in the
tissues and organs to cause the death of a large number of cells and consequently
to impair tissue or organ functions early after exposure. The severity
of injury depending on the absorbed dose according to an s-shaped dose-response
curve might be manifested in the various syndromes of radiation illness,
i.e. the bone marrow, the gastro-intestinal and the central nervous system-vascular
syndromes. The effects can be detected by laboratory and clinical techniques.
The stochastic effects might occur following
low doses (below several tens or 100–200 mSv). The probability of
consequences increases with the dose and the relationship between dose
and effect is assumed to be linear. Accordingly, not having a “threshold”
dose a certain risk – albeit very small – can be attributed to any low
dose.
Such late effects might be the development of malignant
(cancerous) diseases and of the hereditary consequences. Here, it has to
be mentioned that in human populations hereditary effects could not be
detected even in the offsprings of the large population of A-bomb survivors
in the first two generations. The possibility of hereditary alterations
is known only from experimental observations in radiation biology.
The model for assessing the detrimental health effects
used for the deterministic effects is the non-linear-threshold (NL-T) model,
while for the stochastic effects the linear-non-threshold “L-NT” one. In
the low dose dilemma the problem raised is whether the use of the
L-NT model is justified to attach any health risks to low doses.
WHAT IS “LOW DOSE”?
Low doses are considered by observations in epidemiology, cellular
radiation biology and microdosimetry. The levels according
to these views are demonstrated in Table
1.
Based on epidemiological data of radiation-induced
cancer occurrences, various authors agree that low dose is below 200 mGy
as under this level the statistical evaluation of data becomes more and
more uncertain (UNSCEAR 1994; Tubiana et. al. 1995; Heidenreich et. al.
1997). Accordingly, based on the frequency of cancer cases the extrapolation
of risks from high doses to low ones is not justified.
Certain cellular reactions like enzyme inductions,
DNA-repair processes, adaptive responses, chromosome aberrations, etc.
could already be observed between 10 and 100 mGy by various sensitive assay
techniques (Table
2). Therefore this dose-range is considered low. In general, the
doses causing fully recoverable cellular damages or alterations might be
considered low doses in the cell biology.
In microdosimetry the low dose is defined
when 20 per cent of targets, i.e. cells in a tissue are hitted (Bond et
al., 1988; Feinendegen et al., 1988; Booz and Feinendegen, 1988).
Table
1.
Dose ranges considered low in various approaches to biological effects
of ionizing radiations
| Approaches |
mGy, mSv |
References |
For stochastic effects
as carcinogenicity
for gamma and x-rays
for neutrons
For cellular reactions
By microdosimetrical
consideration |
200
200
50
10–100
when less than 20 % of “gross sensitive volume – GSV”
(F target) will be hit once |
NCRP 1980
ICRP 1991
Fry 1996
UNSCEAR 1994
UNSCEAR 1994
See Table
2
Feinendegen 1990 |
For comparison:
The avarage natural background
in a year
in the life-time of a person
“Insignificant individual dose”
“De minimis” dose |
3
50–200
0.01
0.01 |
Webb and McLean 1977
Kocher 1987 |
Among the low dose radiation-induced cellular alterations
recently special interest has been focussed toward the hormesis and
adaptive responses. Although these phenomena, i.e. inducing stimulatory
or beneficial effects are more and more targets for research, no direct
evidence is available for their possible impact on human radiation protection.
It is also worth mentioning that when individual
radiosensitivity of persons was studied through the frequencies of radiation-induced
lymphocytic micronuclei following in vitro irradiation of individual blood
samples, below 200 mGy the responses were found to be unrelated to the
absorbed dose (Köteles et al. 1997). These data suggest the importance
of other factors in individual sensitivity besides the dose. On the other
hand, the data point to the existence of dose-effect modifying biological
factors making the response statistics uncertain like in epidemiology below
the same dose range.
In the foregoings the dose range was given in mGys.
It has to be recalled that the natural background from cosmic and terrestrial
sources is appr. 3 mGy in world average. This level means that 1 hit offends
each of our cells once in a year! For comparison of considerations on low
doses it has to be noted that earlier the opinion was expressed that 10
µSv for an individual is an “insignificant dose” (Webb and McLean
1977) or with an other wording 10 µSv is a “de minimis dose” (Kocher
1987). The expression comes from the language of jurisprudence, i.e. “De
minimis non curat lex”, i.e. – the law does not care with minimal causes
or effects. The limitations and consequences of these expressions have
been outlined and criticized (Lindell 1989), still 10 mSv per year is considered
to be a dose to any member of the public the source or practice of which
may be exempted from requirements of radiation protection standards without
further consideration (IAEA-IBSS 1996). At dose levels when “the collective
dose committed by one year of performance of the practice is no more than
about 1 man-sievert or an assessment for the optimalization of protection
shows that exemption is the optimum option” the risk assessments based
on the collective dose is not justified (IAEA-IBSS 1996).
Table
2.
Examples for cellular responses and alterations provoked by low
doses
| Response/alteration |
Dose-range
mGy |
References |
Free radicals
granulocyte oxidant production increases
superoxide dismutase in spleen increases
oxidative stress increases
Cellular responses
Lymphocyte mitogenic stimulation by lectins
increases
Thymidin kinase activity
Phospholipase C,
Adenylatecyclase
Guanilatecyclase increases
Rosette formation
CHO-CD2+ fenotype alteration
Spleen colony formation stimulation
Mutagenic alterations
lymphoblast 6-thioguanin
resistance (6-TG') mutant formation increases
Nuclear structure
chromatin conformation alteration
Cytogenetic alteration
micronucleus formation
Cell membrane stucture and function
lipid composition changes
antioxidant capacity decreases
micromorphological alteration
lectin binding alteration
Adaptive response develops
Tumor metastasis reduced |
0,1–1
100
50
10–40
10
2,5
10
6
10
40–250
>10
10–100
10–100
250
5–10
200 |
Vicker et al. 1991
Yamaoka et al. 1990
Emerit 1997
Makinodan and James 1990
Nogami et al. 1994
Feinendegen et al. 1988
Krymskx-Ruda et al. 1992
Kitsiou et al. 1993
Rozhdestvensky and Fomicheva 1995
Grosovsky and Little 1985
Belyaev and Harms-Ringdahl 1996
Köteles et al. 1997
Petcu et al. 1997
Bojtor and Köteles 1998
Burlakova 1992
Burlakova 1992
Köteles 1979
Kubasova et al. 1981 a,b
Köteles 1982
UNSCEAR 1994
Mosoi and Sakamoto 1990 |
Beside the doses the biological responses depend
also on the dose rates. At low doses the decreasing dose rate results in
the reduction of biological damages, therefore, the ICRP has introduced
the “dose-dose rate effectiveness factor DDREF” in the assessment of risk,
when the absorbed dose is below 200 mGy and when the dose rate is less
than 100 mGy per hour at higher ones. Though in cases of various detrimental
effects the value of DDREF might vary, the value of 2 was selected (ICRP
1991).
As low dose rates the value of 0,1 mSv per minute
was considered by the (UNSCEAR 1994) for low LET radiations. The natural
radiation background dose rate is 1–3 mSv per year.
THE MAIN ARGUMENTS RAISED AGAINST THE L-NT MODEL
The arguments of those who are opposing the L-NT modell can be summarized
as follows though not necessarily including all the available reasoning:
-
Below 200 mSv no cancer cases were found in a significantly increased frequency
among the A-bomb survivors (UNSCEAR 1994; Duport 1996; Little and Muirhead
1996, Heidenreich et al. 1997a and b; Pierce and Preston 1997).
-
No higher risk could be detected at high natural background areas though
they might be 3–10-times higher than the average level (Yalow 1986; Kondo
1993; UNSCEAR 1994).
-
The carcinogenesis is not a first order kinetic process, i.e. the hit of
cellular genes at sensitive sites or the malignant transformation of one
or a few cells does not lead necessarily to the clinical manifestation
of a malignant disease (Duport 1996; Cox 1996; Pierce 1997; Mendelsohn
1997).
-
The repair of a few single strand breaks (SSB) in the DNA or double strand
breaks (DSB) following a damage by low doses should not mean an overloading
for repair capacity of a cell when the metabolism of cellular DNA and the
repair of damages induced by other endogenous or exogenous factors is running
with much higher intensity,
-
a real threshold might appear when the latency period is long enough compared
with the life time of an individual,
-
by low dose exposures no detectable biological or health effects are provoked
(Bond et al. 1996),
-
the L-NT model is for regulatory purposes as a tool for risk assessment
but it is not verified scientifically or statistically in epidemiology
(Becker 1997).
THE MAIN ARGUMENTS RAISED FOR THE L-NT MODEL
The arguments of those who are in favour of keeping this model further
as valid for the risk assessment are mainly the followings:
-
The linearity is known and it has a great tradition in radiation biology
since the epoch-making experimental results of Muller (1928) on the genetic
effects on Drosophila.
-
For the stochastic effects the L-NT modell is used and recommended by the
ICRP and it is widely used in assessment of health detriment.
-
In cellular radiation biology including observations on the survival of
cells in culture, formation of cytogenetic aberrations and mutations many
results can be fitted to a linear dose-response relationship or linear-quadratic
one when the investigations are extended to higher doses, e.g. appr. above
1 Gy.
-
Recent data indicate significant increase of risk for thyroid cancer, breast
cancer and malignancy to following in utero irradiations also in
the range between 10 and 100 mGy (Clarke 1996; Sobolev et al. 1997; Miller
and Boice 1997; Delongchamp et al. 1997).
-
Even among the A-bomb survivors the risk of cancer increased already above
50 mGy significantly, though it is obvious that the risk below 100 mGy
is really small (Pierce et al. 1996).
-
Among the “liquidators” of the Chernobyl accident site increases of malignant
tumours of the digestive system were observed up to 1995. The average dose
of the studied cohort was 108 mGy (Ivanov et al 1998).
-
It is also repeatedly mentioned that the consequences of DSB if not repaired
are more serious than the consequences of SSBs.
CONCLUSIONS
It is foreseen that the multisided debate will be continued but at this
stage some views can be delineated as conclusions:
Further research and investigations both
on the cell biological as well as on the epidemiological aspects of health
consequences of low doses are necessary (Little 1990; Clarke 1991; Oftedal
1991; Modan 1993; Schull 1996).
It has to be realized that a biological response
itself experienced following rather low doses does not mean detrimental
health consequences.
The L-NT model might be too conservative and unjustified
but at present it seems to be safe enough to ensure the safe application
and uses of ionizing radiation and nuclear energy.
The rejection of the L-NT modell and the acceptance
of a threshold in cases of stochastic effects would raise many questions
concerning the regulatory actions. These foreseen questions like the safe
thresholds for late effects, for different population groups, for various
practices, etc. are hardly answerable at the moment. It seems to be easier,
however, to reach an agreement on the level of acceptable risk instead
of the risk threshold dose.
The debate, however, reveals the important aspects
of the risk assessment and risk perception of the society. The professionals
themselves are grouped – certainly not halved as the opponents of L-NT
are probably still in minority. It is, however, a warning not to overestimate
the risk due to the use of ionizing radiation and nuclear technology especially
when proper radiation protection services are provided. The use of fire
is unavoidable for mankind, though also dangerous, therefore the human
society elaborated ways and means of fire protection. A similar attitude
is needed for the use of radiation.
And last but not least the society at large has
to perceive the various risks in an industrialized country in a comparative
way. In this case, the real risks due to radiation are not at the first
place as certain interviewed student groups have assessed it (UNEP 1985;
Faragó and Engländer 1987).
Professionals in occupational and environmental
medicine might also assist in releasing the people from irrational anxieties,
the latter being also an etiological contributor for many diseases.
REFERENCES
The References has intended to give only a few references out of the
vast literature for the first orientation of the reader.
Becker, K. (1997). “Threshold or no Threshold, that is the Question.”
Radiat. Prot. Dosim., 71, 3–5.
Belyaev, I. Y., and Harms-Ringdahl, M. (1996). “Effects of gamma rays
in the 0,5–50-cGy range on the conformation of chromatin in mammalian cells.”
Radiat. Res., 145, 687–693.
Bojtor, I., and Köteles, G. J. (1998). “Low dose response analysis
through a cytogenetic end-point.” Centr. Europ. J. Occup. Environm. Med.,
4:15–24.
Bond, V. P., Feinendegen, L. E., and Booz, J. (1988). “What is a ‘low
dose’ of radiation?” Int. J. Radiat. Biol., 53, 1–12.
Bond, V. P., Wielopolski, L., and Shani, G. (1996). “Current misinterpretations
of the linear no-threshold hypothesis.” Health Phys., 70, 877–882.
Booz, J., and Feinendegen, L. E. (1988). “A microdosimetric understanding
of low-dose radiation effects.” Int. J. Radiat. Biol., 53, 1, 13–21.
Burlakova, E. B. (1992). “Low dose of irradiation. Ficture of action.”
Europ. Soc. Radiat. Biol., Erfurt. Abstracts.
Clarke, R. H. (1996). “The threshold controversy.” Radiol. Prot. Bull.
No. 178. National Radiological Protection Board, Didcot, UK.
Clarke, R. H. (1991). “Problems of obtaning risk estimates at low doses.”
Radiol. Prot. Bull., National Radiological Protection Board, No. 121. 10–13.
Cox, R. (1996). “Fundamental biological processes in radiation tumorigenesis.”
Rad. Prot. Dosim. 68, 105–110.
Delongchamp, R. R., Mabuchi, K., Yoshimoto, Y., and Preston, D. L. (1997).
“Cancer mortality among atomic bomb survivors exposed in utero or as young
children, October 1950 – May 1992.” Radiat. Res., 147, 385–395.
Duport, P. (1996). “The recommendations of the French Academy of Sciences
on ICRP 60.” Bulletin of the Canad. Radiat. Prot. Assoc., 17, 18–21.
Emerit, I., and further 36 authors (1997). “Oxidative stress and low
dose irradiation.” In: Low doses of ionizing radiation: biological effects
and regulatory control. International Atomic Energy Agency, IAEA-TECDOC-976,
Vienna, pp. 1–4.
Faragó, K., and Engländer, T. (1987). “Perception of risks.
A comparative study with American and Hungarian groups.” In: Kockázat
és Társadalom, ed. Vári A. (in Hungarian). Akadémiai
Kiadó, Budapest, pp. 34–56.
Feinendegen, L. E., Bond, V. P., Booz, J., and Mühlensiepen, H.
(1988). “Biochemical and cellular mechanisms of low-dose effects.” Int.
J. Radiat. Biol., 53, 1, 23–37.
Feinendegen, L. E. (1990). “The cell dose concept: potential application
in radiation protection.” Phys. Med. Biol., 35, 597–612.
Fry, R. J. M. (1996). “Effects of low doses of radiation.” Health Physics,
70, 823–827.
Gonzalez, A. J. (1994). “Biological effects of low doses of ionizing
radiation: A fuller picture.” IAEA Bulletin, 4/1994. 37–45, International
Atomic Energy Agency, Vienna.
Grosovsky, A., and Little, J. B. (1985). “Evidence for linear response
for the induction of mutations in human cells by x-ray exposures below
10 rads.” Proc. Natl. Acad. Sci. USA, 82, 2092–2095.
Heidenreich, W. F., Paretzke, H. G., and Jacob, P. (1997a). “No evidence
for increased tumor rates below 200 mSv in the atomic bomb survivors’ data.”
Radiat. Environ. Biophys., 36, 205–207.
Heidenreich, W. F., Paretzke, H. G., and Jacob, P. (1997b). “Reply to
the ‘Commentary’ by D. A. Pierce and D. L. Preston.” Radiat. Environ. Biophys.,
36, 211–212.
Hosoi, Y., and Sakamoto, K. (1990). “Suppression of artificial lung
metastasis by low doses of total body irradiation.” J. Radiat. Res., 31,
67.
IAEA (1997). “Low doses of ionizing radiation: biological effects and
regulatory control. Contributed papers.” International Atomic Energy Agency
– TECDOC-976.
IAEA-IBSS (1996). “International Basic Safety Standards for Protection
against Ionizing Radiation and for the Safety of Radiation Sources.” International
Atomic Energy Agency, Safety Series No. 115.
ICRP (1991). “1990 Recommendations of the International Commission on
Radiological Protection.” Annals of the ICRP, 21. No. 1–3. 201 p.
Ivanov, V. K., Rastopchin, E. M., Gorsky, A. I., and Ryvkin, V. B. (1998).
“Cancer incidence among liquidators of the Chernobyl accident: solid tumors,
1986–1995.” Health Phys. 74. 309–315.
Kitsiou, P., Sambani, C., and Thomou, H., (1993). “Effects of low-doses
of X-rays on the expression of human cell surface CD2 antigen in CD2+ CHO
cells.” Int. J. Radiat. Biol., 64, 621–626.
Kondo, S. (1993). “Health effects of low-level radiation.” Kinki Univ.
Press, Osaka.
Kocher, D. C. (1987). “A proposal for a generally applicable de minimis
dose.” Health Phys., 53, 117–125.
Köteles, G. J. (1979). “New aspects of cell membrane radiobiology
and their impact on radiation protection.” Atomic Energy Rev. 17,
3–30.
Köteles, G. J. (1982). “Radiation effects on cell membranes.” Radiat.
Environ. Biophys. 21, 1–18.
Köteles, Gy. (1996). “Relationship between doses and effects of
ionizing radiation.” Budapesti Közegészségügy,
28, 306–309. (in Hungarian).
Köteles, G. J., Bojtor, I., Horváth, GY., and Kubasova,
Tamara (1997). “Low dose effects detected by micronucleus assay in lymphocytes.”
In: Low doses of ionizing radiation: biological effects and regulatory
control. International Atomic Energy Agency, IAEA-TECDOC-976, pp. 55–58.
Krymsky-Ruda, V. P., Narimanov, A. A., and Kuzin, A. M. (1992). “The
activation of sensitivity of membrane receptors to natural effectors by
low dose gamma-radiation.” In: Low dose irradiation and biological defense
mechanisms. (Eds. T. Sugahara, L. A. Sagan, T. Aoyama), Elsevier Sci. Publ.,
pp. 327–329.
Kubasova, T., Varga, L. P., and Köteles, G. J. (1981). “Surface
alterations of mammalian cells upon ionizing radiation as detected by a
lectin-binding technique. I. Binding of concanavalin A by blood cells of
X-irradiated mice.” Int. J. Radiat. Biol. 40, 175–186.
Kubasova, T., Köteles, G. J., and Varga, L. P. (1981). “Surface
alterations of mammalian cells upon ionizing radiation as detected by a
lectin-binding technique. II. Binding concanavalin A by human blood cells
X-irradiated in vitro.” Int. J. Radiat. Biol. 40, 187–194.
Lindell, B. (1989). “Comments on various views on the concept of ‘de
minimis’.” Health Phys., 57, 211–212.
Little, M. P., and Muirhead, C. R. (1996). “Evidence for curvilinearity
in the cancer incidence dose-response in the Japanese atomic bomb survivors.”
Int. J. Radiat. Biol. 70, 1, 83–94.
Little, J. B. (1990). “Low dose radiation effects: interactions and
synergism.” Health Phys., 59, 49–55.
Makinodan, T., and James, S. J. (1990). “T-cell potentation by low dose
ionizing radiation: possible mechanisms.” Health Phys., 59, 29–34.
Mendelsohn, M. L. (1997). “Multistage processes of radiation induced
malignancies: mechanisms of initiation, promotion and progression.” Proc.
Intl. Conf. On Low doses of ionizing radiation. IAEA, Vienna, in press.
Miller, R. W., and Boice, J. D., Jr. (1997). “Cancer after Intrauterine
Exposure to the Atomic Bomb.” Radiat. Res., 147, 396–397.
Modan, B. (1993). “Low dose radiation carcinogenesis. Issues and interpretation.”
Health Phys., 65, 475–780.
Mossman, K. L., Goldman, M., Massé, F., Mills, W. A., Schiager,
K. J., and Vetter, R. J. (1996). “Radiation risk in perspective.” Health
Phys., Soc. Newsletter, 24, 2–3.
Muller, H. J. (1928). “Artificial transmutation of the gene.” Science,
66, 84–87.
NCRP (1980). National Council on Radiation Protection and Measurements.
“Influence of dose and its distribution in time on dose-response relationship
for low-let radiations.” NCRP Report No. 64. Bethesda.
Nogami, M., Huang, J. T., Nakumara, L. T., and Makinodan, T. (1994).
“T-cells are the cellular target of the proliferation-augmenting effect
of chronic low-dose ionizing radiation in mice.” Radiat. Res., 139,
47–52.
Oftedal, P. (1991). “Biological low-dose radiation effects.” Mutat.
Res., 258, 191–205.
Pierce, D. A., Shimizu, Y., Preston, D. L., Vaeth, M., and Mabuchi,
K. (1996). “Studies of the mortality of atomic bomb survivors. Report 12,
Part. I.” Cancer. 1950–1990., 146, 1–27.
Pierce, D. A. (1997). “Multistage processes of radiation induced malignancies:
mechanisms of initiation, promotion and progression.” Proc. Intl. Conf.
On Low doses of ionizing radiation. IAEA, Vienna, in press.
Pierce, D. A., and D. L. Preston (1997). “No evidence for increased
tumor rates below 200 mSv in the atomic bomb survivors’ data.” Radiat.
Environ. Biophys. 36, 209–210.
Petcu, I., Savu, D., Moiroi, N., and Köteles, G. J. (1997). “In
vitro and in vivo effects of low dose HTO contamination modulated by dose
rate.” In: Low doses of ionizing radiation: biological effects and regulatory
control. International Atomic Energy Agency. IAEA-TECDOC-976, pp. 312–315.
Rozhdestvensky, L. M., and Fomicheva, E. I. (1995). “The estimation
of haematopoietic stem cell reaction to low level ionising radiation.”
Radiat. Prot. Dosim., 62, 49–51.
Schull, W. J. (1996). “Radioepidmeiology of the A-bomb survivors.” Health
Phys., 70, 798–803.
Streffer, C., and Tanooka, H. (1996). “Biological effects after small
radiation doses.” Int. J. Radiat. Biol. 69, 269–272.
Sobolev, B., Heidenreich, W. F., Kairo, I., Jacob, P., Goulko, G., and
Likhtarev, I. (1997). “Thyroid cancer incidence in the Ukraine after the
Chernobyl accident: comparison with spontaneous incidences.” Radiat. Environ.
Biophys. 36, 195–199.
Tubiana, M. (1991). “Effect of low dose irradiation. Reports of the
French and US Academy of Sciences.” Bull Cancer., 78, 11–17.
Tubiana, M., Latarjet, R., and Lafuma, J. (1995). “Not so stupid.” New
Scientist, 148, 56.
UNEP (1985). Radiation Doses, Effects, Risks., United Nations Environmental
Programme. Nairobi, Kenya 1985.
UNSCEAR (1994). “Sources and effects of ionizing radiation.” United
Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR,
New York 1994.
Vicker, M. G., Bultmann, H., Glade, U., and Häfker, T. (1991).
“Ionizing radiation at low doses induces inflammatory reactions in human
blood.” Radiat. Res., 128, 251–257.
Webb, G. A. M., and McLean, A. S. (1977). “Insignificant levels of dose:
a practical suggestion for making decisions.” Int. Rad. Prot. Assoc., Paris,
Proceedings, pp. 3–9.
Yalow, R. S. (1986). “Biologische Auswirkungen kleiner Strahlendosen.”
Bulletin ASE/UCS, 77, 96–102.
Yamaoka, K., Edamatsu, R., and Mori, A. (1990). “Study on low dose radiation
effects – SOD activities and peroxylipids of brain, liver and immune organs
in rats.” J. Radiat. Res., 31, 67.
| Vol.4. No.2 |

